Description
France COVID-19 Pandemic Mitigation Products Market – 2020-2024 Report
On the backdrop of the need for essential medical products and services, a new market has emerged, the COVID-19 pandemic mitigation products market. This France COVID-19 pandemic mitigation cumulative 2020-2024 market* is worth $67-87 Billion.
 |
(*) Market size is year and scenario dependent.
To adhere to our high standards of research, as nobody can forecast the future of the pandemic, we include in the report two scenarios:
- Optimistic scenario– assumes (among other things) that mass vaccination will commence by July 2021
- Conservative scenario– assumes (among other things) that no mass vaccination will be available until July 2024
Why trust this report?
- The team which created this report was led by ex-executives of the medical industry and bio-security experts, who wrote this report with the COVID-19 industry executives in mind
- As the COVID-19 pandemic knowledge changes all the time, we update the report once a month
- The team has published since 2006 36 Pandemic related reports
- Team members managed since 1979 three medical systems & instrumentation R&D subsidiaries in France
Bottom Line: While other COVID-19 reports are written (at best) by MBAs, this report is published by professionals for experts
2023 France COVID-19 Pandemic Mitigation Market Share (Scenario A)
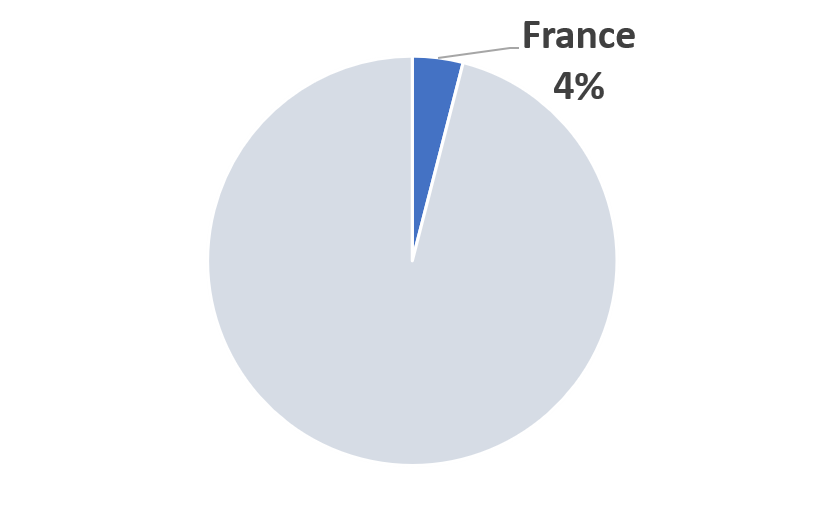 |
According to the report:
As this report is published France has reported 160,377 COVID-19 infected and 29,640 fatalities. Following a long lockdown of millions, France is recovering from a first wave of the COVID-19 outbreak, with many sectors gradually returning to normal levels of activity. Factories are restarting production and consumers are beginning to spend again. However, the crisis has had a dramatic and lingering impact on the national economy.
 |
France has one of the world’s best health care systems and that system is facing its severest test ever, and whether it succeeds will say much about the ultimate adequacy of a well-funded, well-equipped, and broadly accessible national treatment plan.
If France’s experiment in confining its citizens less rigorous than the Chinese, more precocious than the Italian, far more organized than the American yields the hoped-for flattening of the curve, it would be vindication not just for the underlying system, but for a Western democracy’s organized effort to combat the coronavirus. The verdict is still weeks away.
France spends more on health than most of its developed-world peers, offers world-beating access to doctors at less cost, and encourages all its citizens, through universal government-funded coverage, to keep track of their conditions. It has twice the number of intensive care beds that Italy has.
Overstretched hospitals in Alsace, the hardest-hit region by far, have had to send out patients by military planes to less affected regions, and even in a “medicalized” train. A conference of 2,000 evangelical Christians at Mulhouse in February, where an unknown but significant number of those there were infected, has had the effect of a coronavirus bomb, first on Alsace, and then on all of France, as the participants spread throughout the country.
For weeks, COVID-19 infected France believed it could escape Italy’s fate even as it kept a wary eye on its neighbor. The initial measures were limited closing schools in the rural Paris exurbs and in the northwestern region of Brittany, where some cases had been noted, and banning gatherings of more than 1,000. With the lockdown action, France put itself in a more favorable position than Italy. The confinement order began when just 148 were dead. The Italian government, by contrast, waited until the death toll was over 800 before ordering a national lockdown.
There has been resistance to the confinement in the immigrant suburbs, where restless residents are crammed into inhospitable tower blocks. Police officers have already handed out tens of thousands of fines.
The number of hospitalized patients and patients in intensive care has been steadily decreasing over the past few months in France, and the number of new deaths each day has fallen to levels not seen since mid-March.
Weeks have passed since authorities began lifting the months-long lockdown, but the government warned restrictions could be reimpose if the number of cases rose again.
All restrictions on internal travel have been lifted, schools and shops are continuing to reopen, and restaurants were the latest to lift their shutters, with restrictions. However, public gatherings of more than 10 people are still banned as are most large-scale events, like music festivals. Wearing masks on public transportation is now mandatory in certain areas.
The government has also ramped up its ability to identify and isolate new cases and trace their contacts. A few clusters have emerged in the weeks since the lockdown lifted, but they do not seem to have led to widespread contamination.
France has a universal health care system, but the virus put both under serious strain. When the outbreak started, the government has faced criticism, especially for its handling of mask shortages. Few have credited the government for beating back the virus. Some, frustrated with an “infantilizing” approach to the lockdown, tested the limits of the restrictions.
France is one of a few countries in Europe that includes COVID-19 deaths in retirement or nursing homes in its country-wide daily death tallies, meaning the overall picture of mortality is more accurate. But the data skewed higher in the early weeks of April when previously unrecorded deaths were suddenly added to the national tally.
Generally speaking, France was late to increase its testing capacities compared to countries like Germany. The national public health authority has acknowledged that the reported number of test-confirmed cases was lower than the real number of cases.
France has centralized official coronavirus-related information and documents like the waivers needed for personal outings on a website. The government also releases daily statistics on the outbreak here. Key numbers and more detailed breakdowns are also available via the national public health authority.
Leading healthcare vendors like GE Healthcare, Philips, IBM and J&J are among the companies that participate in the race to gain a leading market share in the country market.
France Healthcare, ICUs and Vaccines Outlay Statistics (2019)
 |
Sources: IMF, WHO, OECD, World Population Prospects (2019 Revision)
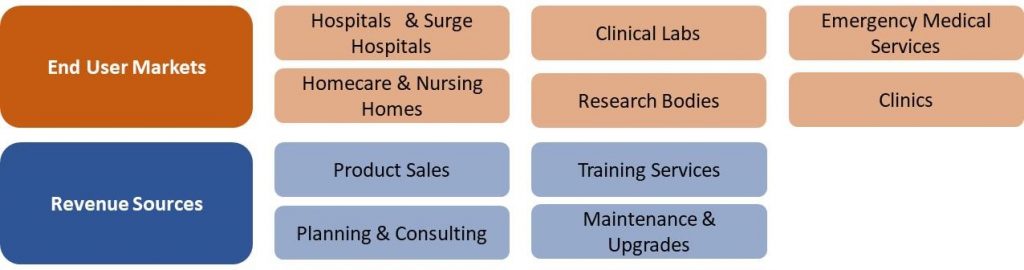
France COVID-19 Market Segmentation Vectors
6 End User and 4 Revenue Source Markets:
 |
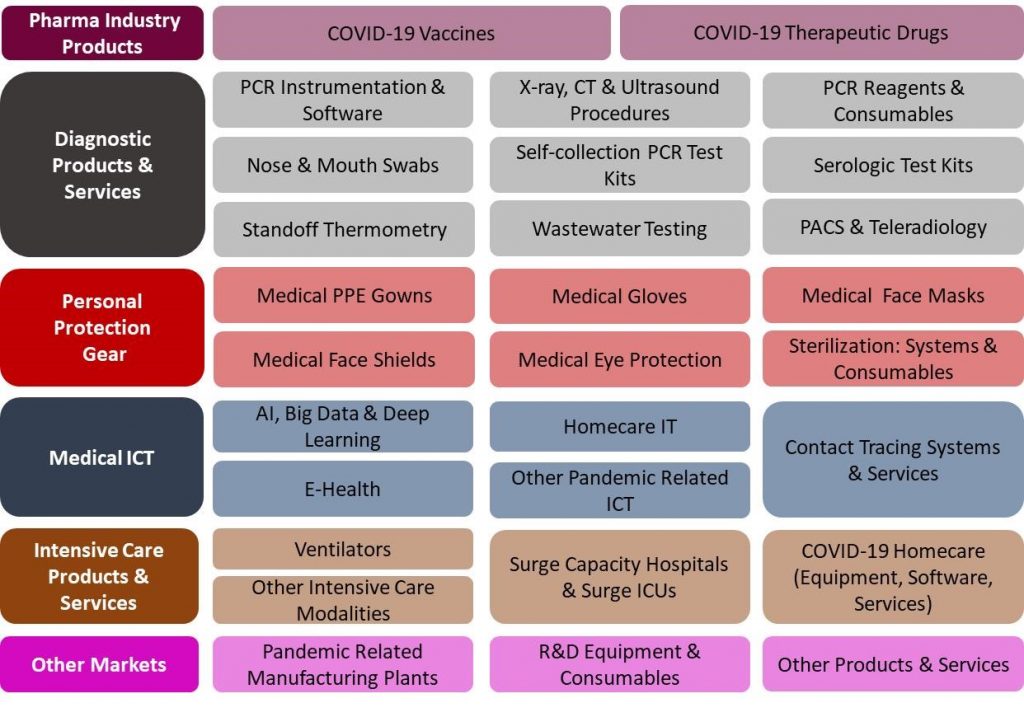
29 Products and Services Markets:
 |
France’s COVID-19 pandemic mitigation products market is driven by the demand for the following:
- COVID-19 medications
- COVID-19 Informatics
- New ICU beds
- COVID-19 medications
- COVID-19 Informatics
- COVID-19 Vaccines
- New ICU beds
- Ventilators
- Electronic Contact Tracing
- Surge Hospitals
- PPE
- Invasive ventilators
- COVID-19 medical imaging services
- Training of ICU and ER personnel
- Strategic National Stockpile
- Pandemic related turnkey manufacturing plants
and the following factors:
- France invests heavily on PCR and serologic tests. Having said that, national health authorities in France do not provide regional data for confirmed cases of the virus
- The growing size of coronavirus related information and mounting complications of datasets, and the need saved by financial resources
- Advanced AI and machine learning have a high demand among general practitioners and others who are not coronavirus ICU experts
- France pharma and biotech companies research and development of coronavirus vaccines and medications
This 450 -page market report is the most comprehensive review of the French COVID-19 market available today. The objective of this report is to provide today’s strategic decision-makers with an expert 360-degree, time-sensitive, detailed view of this interconnected market.
The France COVID-19 Pandemic Mitigation Products Market – 2020-2024 report presents a thorough market analysis of 29 products & services, 6 end users, 4 revenue source markets. Furthermore, the report provides updated extensive data of 37 key vendors.
Why Buy this France COVID-19 Pandemic Mitigation Products Market Report?
A. Questions answered in this report include:
- What is the French COVID-19 Market size and what are the forecast trends during 2020-2024?
- What are the most attractive business opportunities?
- What drives the customers to purchase solutions and services?
- What are the French COVID-19 Market trends?
- What are the challenges to market penetration & growth?
B. French COVID-19 market size data is analyzed via 5 independent key perspectives. With a highly fragmented market we address the “money trail” – each dollar spent in the French COVID-19 market is analyzed and crosschecked via 3 orthogonal viewpoints:
- By 29 Products and Services:
|
|
- By 6 End User Markets:
|
- By 4 Revenue Source Markets:
|
C. Detailed market analysis frameworks for each of the market sectors are provided, including:
|
D. The report provides an updated extensive data of the leading 37 companies (including companies’ profile, recent annual revenues, COVID-19 mitigation activities & products and contact information).
E. The report includes over 2300 links to the COVID-19 pandemic mitigation community information sources
F. The report mentions > 450 Vendors including the following:
Moderna, IBM, 3M Company, Philips NV, Johnson & Johnson, Roche, Nvidia Corporation, Thermo Fisher Scientific, Siemens, Toshiba, Google, Samsung Electronics Co, GE Healthcare, Medtronic, MedWhat, MedyMatch, Merck, Metabiota, Micron Technology, Infermedica, Infervision, Inovio Pharmaceuticals, Microsoft Corporation, Mindshare Medical, Morpheo, Pfizer, Philips NV, Maxim Biotech, Honeywell Safety Products, Philips Healthcare, Siemens, Recursion Pharmaceuticals, Fujifilm Holdings Corporation, 3Scan, Abbott , AbCellera, Advenio Technosys, Agfa Healthcare, Agilent Technologies, AiCure, Aindra, Allscripts Healthcare Solutions, Amara Health Analytics, Amazon , analyticsMD, Apixio, Apple, Arterys Inc., Atlas Wearables, Atomwise, Avalon Nutrition VITL, Babylon Health, Bay Labs, Behold.ai, benevolent.ai, BIOBEATS, BlueDot, Bollé Safety, Bullard, Buoy Health, Care Angel Wearables QorQL, Careskore, Clinithink, Cloud Pharmaceuticals, CloudMedx, CureMetrix Mental health Ginger.io, Cyrcadia, Deep 6 AI, Deep Genomics, Dell Technologies Inc., Delta Plus Group, Desktop Genetics Virtual mate Ada Health, DreaMed Diabetes, Dupont, EaglEyeMed, Eli Lilly, Encon Safety Products, Enlitic, EnsoData, Entopsis, Envisagenics Research iCarbonX, ERB Industries Inc., Ergodyne, Essilor of America, Flashback Technologies, Flow Health, Ford, Freenome, Frequency Therapeutics Inc., Gateway Safety Inc., General Motors, General Vision, Gentex Corporation, Gibco, Gilead Sciences, Globavir Biosciences, Healint, Health Fidelity, HealthNextGen, HexArmor, Hindsait, Imagen Technologies, Imagia Cybernetics, Inside DNA, InSilico Medicine, Intel Corporation, Intendu, Invitrogen, Ion Torrent, Ironwear, Jvion, Kapa Biosystems, Keen Eye Technologies, Kimberly-Clark Professional, Lexmark International Inc., LifeGraph, Lucina Health, Lumiata, Lunit, Lytics, Magnea, Maxwell MRI, McKesson Corporation, MCR Safety, Medal, Medalogix, Medasense, MedAware, Niramai Health Analytix, Novarad Corporation, NuMedii, Numerate, Nuritas Pharma Turbine, Oncora Medical, Ovuline, PeerWell, PhysIQ, Precision Health Intelligence, Predible Health, Profility, Proscia, pulseData, Pyramex Safety, Qualaris Healthcare Solutions, Qualcomm Incorporated, Qualcomm Incorporated, Qure.Ai, Radians Inc., Roam Analytics, RxPREDICT, Safety Optical Service Ltd, Sanofi, Saykara, Sellstrom Manufacturing Company (SureWerx), Sense.ly, Sensory Inc., Sigma-Aldrich Corp., Skymind Inc., Vir Biotechnology Inc., VisionAid Inc., WuXi Biologics, Xilinx Inc. and more.
G. France COVID-19 market report includes 5 appendices:
- Appendix 1: Differences & Similarities between Common Flu and Coronavirus
- Appendix 2: COVID-19 Tests
- Appendix 3: Abbreviations
- Appendix 4: Glossary
- Appendix 5: Bibliography
COVID-19 pandemic mitigation market research team
The team which composed this report brings 43 years of hands on record in the development and commercialization of healthcare products including: antibody antigen identification, E-health, decontamination and biosecurity, PACS, teleradiology, PPE, computerized tomography, ultrasound, electron microscopy, medical devices and more. Our team members bring long term relations with the U.S. FDA and CDC as well as the EU CE and other national medical regulatory agencies.
As early as January 20, 2020 we recruited all our analysts to research the COVID-19 pandemic mitigation related products purchases. We interviewed hundreds of experts, participated in more than 95 conferences and webinars, reviewed more than 1,500 publications and interviewed executives of more than 65 pandemic related companies.





























