Description
US COVID-19 Pandemic Mitigation Products Market – 2020-2024 Report
On the backdrop of the need for essential medical products and services, a new market has emerged, the COVID-19 pandemic mitigation products market. This US COVID-19 pandemic mitigation cumulative 2020-2024 market is worth $510 Billion (scenario B).
 |
(*) Market size is year and scenario dependent.
To adhere to our high standards of research, as nobody can forecast the future of the pandemic, we include in the report two scenarios:
- Optimistic scenario– assumes (among other things) that mass vaccination will commence by July 2021
- Conservative scenario– assumes (among other things) that no mass vaccination will be available until July 2024
Why trust this report?
- The team which created this report was led by ex-executives of the medical industry and bio-security experts, who wrote this report with the COVID-19 industry executives in mind
- As the COVID-19 pandemic knowledge changes all the time, we update the report once a month
- The team has published since 2006 36 Pandemic related reports
- Team members managed since 1974 medical systems factories, sales and service & R&D departments in Michigan, New Jersey and Massachusetts.
Bottom Line: while other COVID-19 reports are written (at best) by MBAs, this report is published by seasoned professionals for experts
 |
The USA Healthcare Spending vs. other G-20 countries
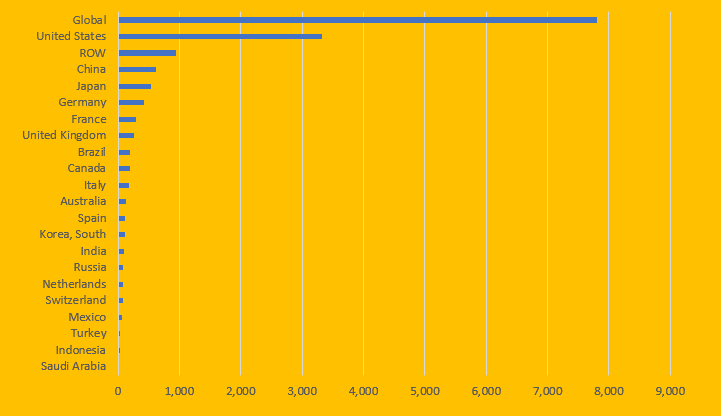 |
By 2019, U.S. healthcare spending stood at $3.3 trillion, more than 42% of global healthcare expenditures.
By March 2020 Congress approved an unprecedented $2.2 trillion COVID-19 first relief package. It includes $161 Billion to the VA, CDC, FDA, Medicare, the Strategic National Stockpile and COVID-19 Vaccines. It does not cover the monthly deficit of $50 Billion that the Hospitals Managers Association reported.
USA has one of the biggest regional inequalities. Big towns on the East Coast were hit hard by COVID-19 in March and April, other American states imposed a locked down policy fast enough to curtail outbreaks. The rate of coronavirus deaths per 100,000 people in the U.S. is lower than in several European countries.
Having said that, by July America suffered from a coronavirus second wave. In several states it took a month to update death certificates, which means that the initial numbers they publish for a given time are considerable undercounts, leading to the false sense of deaths.
 |
The U.S. Healthcare, ICUs, and Vaccines Outlay Statistics (2019)
 |
Sources: IMF, WHO, OECD, World Population Prospects (2019 Revision)
According to the report, despite the huge size of the U.S. Healthcare sector, the U.S. became the global leading COVID-19 victim – why?
- The Federal and State Pandemic management got politicized.
- The administration was late to stop international travel.
- The White House has no COVID-19 war strategy.
- The administration excluded the authority of CDC which was established 77 years ago with one mission: “Mitigate the Spread of Diseases”
- The federal government has limited authority. For example, it cannot force a nationwide lockdown; this is the authority of the 50 states.
- Millions of infected low-income individuals and others, not covered by any medical insurance, refrained from seeking medical care when needed.
- Three years of budget cutting left agencies like the Strategic National Stockpile, CDC, FDA, and others unprepared for a major pandemic.
 |
The USA is and will continue to be the largest COVID-19 mitigation products market because that is the only option it has, and it has unlimited resources to invest whatever it takes to mitigate the pandemic.
The U.S.’s COVID-19 pandemic mitigation products market drivers are:
- The catastrophic state of the pandemic in human suffering and economical cost will turn into a perfect storm by the fall, when the common influenza will coincide with COVID-19. The common flu will infect tens of millions and saturate the COVID-19 mitigation infrastructure (the 2017–2018 flu season was severe, it resulted in an estimated 950,000 hospitalizations and 61,100 deaths)
- The need to invest in all the products and services listed below
- The need to invest in training ICU and 1st responding personnel
- Investments in new ICU units and surge hospitals
- Investments in coronavirus related research and development
- Investments in the expansion of the CDC, FDA, NIH and the Strategic National Stockpile
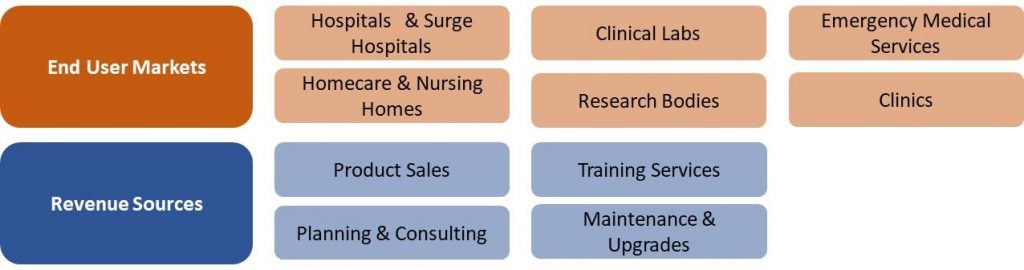
US COVID-19 Market Segmentation Vectors
6 End User and 4 Revenue Source Markets:
 |
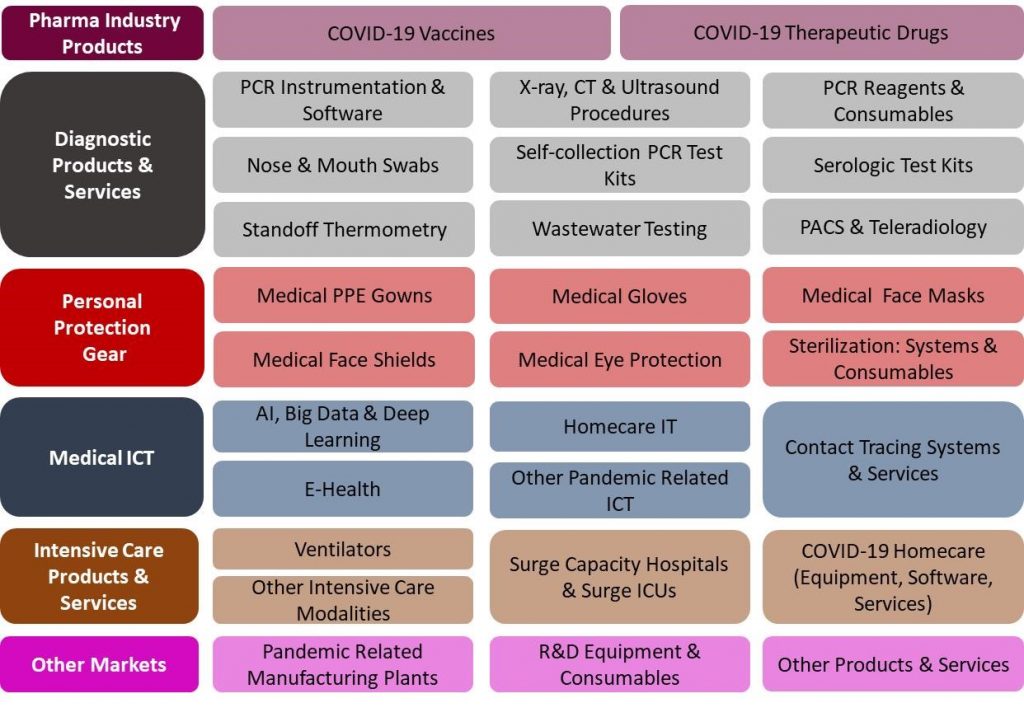
54 Products and Services Markets:
 |
The USA COVID-19 pandemic mitigation products market is driven by the demand for the following:
- PCR tests
- Serologic Tests
- COVID-19 Vaccines
- Ventilators
- Electronic Contact Tracing
- Surge Hospitals
- PPE
- Invasive ventilators
- New ICU units
- COVID-19 medications
- COVID-19 Informatics
- COVID-19 medical imaging services
- Training of ICU and ER personnel
- Strategic National Stockpile
- Pandemic related turnkey manufacturing plants
and the following factors:
- On the backdrop of the first on-going outbreak in the country, and the removal of the lockdown, the country healthcare establishment invests billions to mitigate the next wave of the pandemic
- The growing size of coronavirus related information and mounting complications of datasets, and the need saved by financial resources
- Advanced AI and machine learning haves a high demand among general practitioners and others who are not coronavirus ICU experts
- The country Pharma and Biotech companies research and development of coronavirus vaccines and medications
This 450 -page market report is the most comprehensive review of the American COVID-19 market available today. The objective of this report is to provide today’s strategic decision-makers with an expert 360-degree, time-sensitive, detailed view of this interconnected market.
The US COVID-19 Pandemic Mitigation Products Market – 2020-2024 report presents a thorough market analysis of 29 products & services, 6 end user and 4 revenue source markets. Furthermore, the report provides updated extensive data of 37 key vendors.
Why Buy this US COVID-19 Pandemic Mitigation Products Market Report?
A. Questions answered in this report include:
- What is the American COVID-19 Market size and what are the forecast trends during 2020-2024?
- What are the most attractive business opportunities?
- What drives the customers to purchase solutions and services?
- What are the US COVID-19 Market trends?
- What is the 10 sub-markets size over the 2020-2024 period?
- What are the challenges to market penetration & growth?
B. American COVID-19 market size data is analyzed via 5 independent key perspectives. With a highly fragmented market we address the “money trail” – each dollar spent in the US COVID-19 market is analyzed and crosschecked via 3 orthogonal viewpoints:
- By 29 Products and Services:
|
|
- By 6 End User Markets:
|
- By 4 Revenue Source Markets:
|
C. Detailed market analysis frameworks for each of the market sectors are provided, including:
|
E. The report includes over 2300 links to the COVID-19 pandemic mitigation community information sources
F. The report mentions > 450 Vendors including the following:
Moderna, IBM, 3M Company, Philips NV, Johnson & Johnson, Roche, Nvidia Corporation, Thermo Fisher Scientific, Siemens, Toshiba, Google, Samsung Electronics Co, GE Healthcare, Medtronic, MedWhat, MedyMatch, Merck, Metabiota, Micron Technology, Infermedica, Infervision, Inovio Pharmaceuticals, Microsoft Corporation, Mindshare Medical, Morpheo, Pfizer, Philips NV, Maxim Biotech, Honeywell Safety Products, Philips Healthcare, Siemens, Recursion Pharmaceuticals, Fujifilm Holdings Corporation, 3Scan, Abbott , AbCellera, Advenio Technosys, Agfa Healthcare, Agilent Technologies, AiCure, Aindra, Allscripts Healthcare Solutions, Amara Health Analytics, Amazon , analyticsMD, Apixio, Apple, Arterys Inc., Atlas Wearables, Atomwise, Avalon Nutrition VITL, Babylon Health, Bay Labs, Behold.ai, benevolent.ai, BIOBEATS, BlueDot, Bollé Safety, Bullard, Buoy Health, Care Angel Wearables QorQL, Careskore, Clinithink, Cloud Pharmaceuticals, CloudMedx, CureMetrix Mental health Ginger.io, Cyrcadia, Deep 6 AI, Deep Genomics, Dell Technologies Inc., Delta Plus Group, Desktop Genetics Virtual mate Ada Health, DreaMed Diabetes, Dupont, EaglEyeMed, Eli Lilly, Encon Safety Products, Enlitic, EnsoData, Entopsis, Envisagenics Research iCarbonX, ERB Industries Inc., Ergodyne, Essilor of America, Flashback Technologies, Flow Health, Ford, Freenome, Frequency Therapeutics Inc., Gateway Safety Inc., General Motors, General Vision, Gentex Corporation, Gibco, Gilead Sciences, Globavir Biosciences, Healint, Health Fidelity, HealthNextGen, HexArmor, Hindsait, Imagen Technologies, Imagia Cybernetics, Inside DNA, InSilico Medicine, Intel Corporation, Intendu, Invitrogen, Ion Torrent, Ironwear, Jvion, Kapa Biosystems, Keen Eye Technologies, Kimberly-Clark Professional, Lexmark International Inc., LifeGraph, Lucina Health, Lumiata, Lunit, Lytics, Magnea, Maxwell MRI, McKesson Corporation, MCR Safety, Medal, Medalogix, Medasense, MedAware, Niramai Health Analytix, Novarad Corporation, NuMedii, Numerate, Nuritas Pharma Turbine, Oncora Medical, Ovuline, PeerWell, PhysIQ, Precision Health Intelligence, Predible Health, Profility, Proscia, pulseData, Pyramex Safety, Qualaris Healthcare Solutions, Qualcomm Incorporated, Qualcomm Incorporated, Qure.Ai, Radians Inc., Roam Analytics, RxPREDICT, Safety Optical Service Ltd, Sanofi, Saykara, Sellstrom Manufacturing Company (SureWerx), Sense.ly, Sensory Inc., Sigma-Aldrich Corp., Skymind Inc., Vir Biotechnology Inc., VisionAid Inc., WuXi Biologics, Xilinx Inc. and more.
G. US COVID-19 market report includes 5 appendices:
- Appendix 1: Differences & Similarities between Common Flu and Coronavirus
- Appendix 2: COVID-19 Tests
- Appendix 3: Abbreviations
- Appendix 4: Glossary
- Appendix 5: Bibliography
COVID-19 pandemic mitigation market research team
The team which composed this report brings 43 years of hands on record in the development and commercialization of healthcare products including: antibody antigen identification, E-health, decontamination and biosecurity, PACS, teleradiology, PPE, computerized tomography, ultrasound, electron microscopy, medical devices and more. Our team members bring long term relations with the U.S. FDA and CDC as well as the EU CE and other national medical regulatory agencies.
As early as January 20, 2020 we recruited all our analysts to research the COVID-19 pandemic mitigation related products purchases. We interviewed hundreds of experts, participated in more than 95 conferences and webinars, reviewed more than 1,500 publications and interviewed executives of more than 65 pandemic related companies.




















